Fish Test For Bladder Cancer
Results are intended for use in conjunction with and not in lieu of current standard diagnostic procedures urine cytology as an aid for initial diagnosis of bladder. In 2005 Sarosdy stated.

Performance Of The Urovysion Fish Assay For The Diagnosis Of Malignant Effusions Using Two Cutoff Strategies Rosolen 2018 Cancer Medicine Wiley Online Library
If an imaging test shows enlarged lymph nodes or other possible signs of cancer spread some type of biopsy might be needed to confirm the findings.

Fish test for bladder cancer. Test Usage The UroVysion TM Bladder Cancer Kit Abbott Molecular Inc is designed to detect aneuploidy for chromosomes 3 7 17 and loss of the 9p21 locus via fluorescence in situ hybridization FISH in urine specimens from persons with hematuria suspected of having bladder cancer. When the FISH test is positive and the cystoscopy is Clinical Use. Latest news reports from the medical literature videos from the experts and more.
A quick background - gross hematuria 3 months ago MRI negative showed cyst on. UroVysion is a multiprobe fluorescence in situ hybridization FISH assay that detects common chromosome abnormalities in bladder cancers. 1 2 In addition to urine.
For this study the authors evaluated the effectiveness of multiprobe FISH and urine cytology in detecting urothelial cell carcinoma UCC in the same urine sample. If you have bladder cancer your doctor may order some of these tests to see if the cancer has spread to tissues and organs near the bladder to nearby lymph nodes or to distant parts of your body. For this study the authors evaluated the effectiveness of multiprobe FISH and urine cytology in detecting urothelial cell carcinoma UCC in.
The American Bladder Cancer Societys function is to raise awareness of bladder cancer advocate for research advancement and support bladder cancer survivors. Based on these results the FDA approved Abbotts UroVysionTM DNA probe FISH as a diagnostic tool for bladder cancer in 2005. Ploidy such as that detected by the FISH test are characteristic of bladder cancer.
This test is designed to detect aneuploidy for chromosomes 3 7 and 17 and loss of the 9p21 locus via fluorescence in situ hybridization FISH in urine specimens from persons with hematuria suspected of having bladder cancer. Bladder cancer detection using FISH UroVysion assay UroVysion is a fluorescence in situ hybridization assay that was developed for the detection of bladder cancer in urine specimens. Ad State Of The Art Robotic Surgery To Treat Bladder Cancer.
Presently FISH is used as an aid in the initial diagnosis of bladder can-cer in patients with hematuria. Ad Bladder cancer coverage from every angle. The same pathologist SUGGESTED further study on the one last September but ordered a FISH test for the recent one a 2 weeks ago.
Clinical Significance Bladder Cancer FISH - UroVysion FISH detects aneuploidy of chromosomes 3717 and loss of 9p21 locus. More than 30 urinary biomarkers have been reported for use in bladder cancer diagnosis but only a few are commercially available. Hello Everyone I was hoping to get some insight on the results of a FISH test.
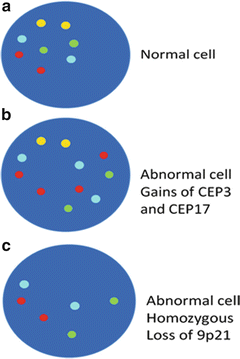
A pivotal multicenter study reported that the overall sensitivity of FISH com-pared to voided urine cytology was 71 and 26. FISH is 42-83 sensitive for detecting pTa and pT1 lesions and 92-100 sensitive for pT2-4 invasive lesions in patients with known bladder cancer while urine cytology yields sensitivities of. It consists of fluorescently labeled DNA probes to the pericentromeric regions of chromosomes 3 red 7 green and 17 aqua and to the 9p21 band gold location.
The same conclusions for the 2 cytologies were drawn. Negative for high grade cancer cells but in both cases small clusters of urothelial cells with mild to moderate atypia. The test is approved as an aid for initial diagnosis of bladder carcinoma in patients with hematuria and subsequent monitoring for tumor recurrence in patients previously diagnosed with bladder cancer.
Minimally Invasive Robotic Surgery To Treat Bladder Cancer. Useful for monitoring bladder cancer and in the initial diagnosis of bladder cancer in patients with hematuria. UroVysion is a multiprobe fluorescence in situ hybridization FISH assay that detects common chromosome abnormal-ities in bladder cancers.
The remainder are still being tested. Should allow physicians to make. First void of the day is.
Due to its high specificity 96 and increased sensitivity Figure 1 the FISH test is useful for early detection of bladder cancer recurrence when used in conjunction with cystos-copy. In October I had a NED cytoscopy. This test is an aid for initial diagnosis of bladder carcinoma in patients with hematuria and subsequent monitoring for tumor recurrence in patients previously diagnosed with bladder cancer.
Use the large open cup in the kit to collect the urine specimen.

Urovysion Test Showing Fluorescence In Situ Hybridization Fish Download Scientific Diagram

Additional Urovysion Information Abbott Molecular
Molecular Diagnosis Of Bladder And Kidney Cancer Oncohema Key

Additional Urovysion Information Abbott Molecular